Abstract
Introduction: BAY 94-9027 is a B-domain-deleted recombinant factor VIII (FVIII) that is site specifically PEGylated with a 60-kDa (2 × 30-kDa) polyethylene glycol (PEG) to extend its half-life. The efficacy and safety of BAY 94-9027 as prophylactic and on-demand therapy for patients with severe hemophilia A were demonstrated in the phase II/III PROTECT VIII trial. This analysis provides an updated assessment of the frequency of bleeds and joint bleeds with BAY 94-9027 prophylaxis during the PROTECT VIII trial and its extension of more than 4 years.
Methods: PROTECT VIII was a partially randomized, open-label trial of 134 males aged 12-65 years with severe hemophilia A (FVIII < 1%) and ≥ 150 previous exposure days (ED). Prophylaxis patients received 25 IU/kg twice weekly for a 10-week run-in period. Patients with ≤ 1 spontaneous joint or muscle bleed during this period were randomized to 45-60 IU/kg every 5 days (E5D) or 60 IU/kg every 7 days (E7D) for the main 26-week study period; patients enrolling after the randomization arms were full, or with ≥ 2 bleeds in the run-in period, received 30-40 IU/kg twice weekly (2×W). Following completion of the main PROTECT VIII trial, patients could enter an extension, continuing BAY 94-9027 prophylaxis on any regimen used in the main study. Prophylaxis patients who switched regimen after 7 days of the extension were analyzed together in a variable frequency group. Overall and joint annualized bleeding rates (ABRs) were calculated based on documentation in electronic patient diaries. Here, we present data for patients receiving prophylaxis.
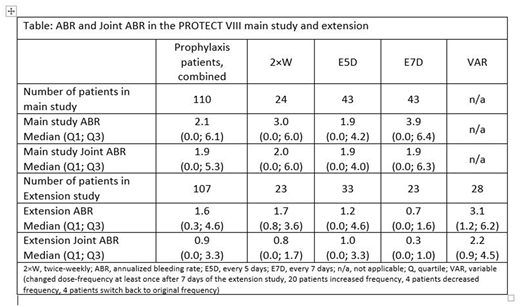
Results: The intent-to-treat population in the PROTECT VIII extension study included 121 subjects (prophylaxis, n = 107; on demand, n = 14) of the 134 patients previously included in the main study (13 did not continue into the extension). At data cut-off (31 January 2018), patients had spent a median time of 3.9 years in the study since enrolment. Patients in the prophylaxis arms had spent a median (range) of 1163 (449; 1579) days in the extension study (approx. 3.2 years), with a median of 211 (96; 318) ED. Most (71) patients had completed 3 years of treatment, 53 had completed 4 years and 33 had completed 5 years of treatment with BAY 94-9027. During the extension, most patients in the 2×W, E5D, or E7D groups did not change regimen (96%, 78% and 65%, respectively). Eleven patients (E5D, 7; E7D, 4) switched to 2×W treatment. Median ABR was 1.6 in prophylaxis patients in the extension, while median joint ABR was 0.9. These values were lower than ABRs during the 6-month main study, of 2.1 and 1.9 for ABR and joint ABR, respectively. In the extension, ABR remained low across all prophylaxis regimens (Table). These values were lower than ABRs for the corresponding treatment groups in the main study (Table). Joint ABR also remained low across extension study patient groups receiving different BAY 94-9027 prophylaxis regimens, and again this was lower than joint ABR in the main study for each group (Table).
Conclusions: In the PROTECT VIII extension study, the majority (79/107, 74%) of prophylaxis patients remained on the same dose-frequency of BAY 94-9027during total time in the extension study. Median ABR and joint ABR were lower over the 3-5 years of extension study than in the main study for prophylaxis patients; this was true for the combined prophylaxis cohort and for the 2×W, E5D and E7D BAY 94-9027 prophylaxis groups. Bleed rates were lowest for patients who stayed in the E7D group. Taken together, these results demonstrate that the 2×W, E5D and E7D dose-frequency options for BAY 94-9027 prophylaxis allow patients a decreasingly low rate of bleeds and joint bleeds once treated with a suitable individual regimen.
Reding:Genentech: Other: Advisory Board. Pabinger:Bayer, Baxalta/Shire, Novo, Pfizer, Biotest, CSL Behring: Honoraria; CSL Behring and Novo.: Research Funding. Maas Enriquez:Bayer: Employment. Ducore:HemaBiologics: Consultancy, Other: investigator, travel support; OPKO: Other: investigator; CSL Behring: Other: investigator; Octapharma: Consultancy, Other: travel support, investigator , Research Funding; Pfizer: Other: investigator; Biomarin: Other: investigator; Spark Therapeutics: Consultancy, Other: investigator; Shire: Consultancy, Other: travel support, investigator; Bayer Healthcare: Consultancy, Other: travel support, investigator.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal